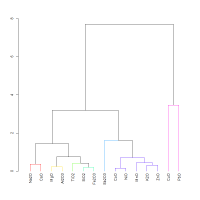
A multivariate analysis was performed of the full chemical data set from Abe et al. (2012), apart from SnO2, which was below the detection limit for the majority of samples. The dendrogram in Figure 8 shows the 18th Dynasty glass from museum collections. As expected NiO, CoO, MnO and ZnO are closely associated, i.e. the cluster is based on their similar patterns of variation. As anticipated from the bivariate plots (Figure 6), alumina has lower co-dependence and is less well associated with all these element oxides, being better associated with the refractory oxides, e.g. MgO and TiO2. CuO and PbO are not well associated with the other components or with each other.

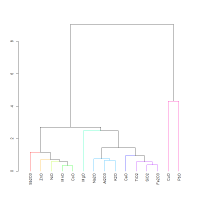
Figure 9 shows the dendrogram for the 19th-20th Dynasty glass from Dahshur. As with the 18th Dynasty museum pieces in Figure 8, NiO, MnO, CoO and ZnO are well associated. However, there are also differences between the two dendrograms: K2O is better associated with Na2O (discussed in Section 4.4) and, Al2O3 is less well associated with MgO and TiO2 (discussed in 4.2) in the 19th-20th Dynasty glass from Dahshur than in the 18th Dynasty glass from museum collections.
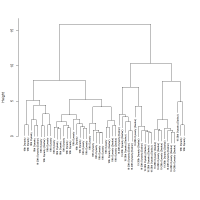
Using the variation matrix for each sample to construct a dendrogram shows that the 18th Dynasty glass and 19th-20th Dynasty glass fall into two distinct groups when the dendrogram is cut high (>10) (Figure 10), apart from one 19th-20th Dynasty and two 18th Dynasty samples. All the 18th Dynasty glass found at Dahshur falls within the main 18th Dynasty group. It will be shown that these groups are a consequence of recycling, rather than different recipes or practices and will be discussed in Section 4.2 and Section 4.3.
To examine this dataset further, Figure 11 shows the relationship between alumina and titania using the 18th Dynasty glass plotted in Figure 10, alongside 18th Dynasty glass and glass from the Ramesside Period (19th-20th Dynasties) found at Dahshur.
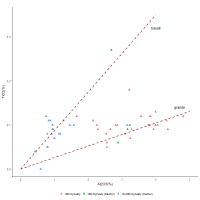
Both the 18th Dynasty data (red triangles) and Ramesside data from Dahshur (blue triangles) show interactions that are relatively linear and potentially converge at the origin. This could suggest that these two components enter the system together. Although a linear regression could have been used to represent these interactions, the lines on the plots are not linear regressions applied to the data but were derived from the average compositions of granite and basalt, the main components of igneous rocks (Table 1; Daly 1914, 169; Parker 1967). In other words, these dashed lines are independent measurements of igneous materials, determined from the average compositions of basalt and granite rocks (Table 1).
| % | SiO2 | TiO2 | Al2O3 | Fe2O3 | FeO | MnO | MgO | CaO | Na2O | K2O | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Granite | 70.47 | 0.39 | 14.90 | 1.63 | 1.68 | 0.13 | 0.98 | 2.17* | 3.31 | 4.10 | 0.24 |
| Basalt | 49.65 | 1.41 | 16.13 | 5.47 | 6.45 | 0.30 | 6.14 | 9.07 | 3.24 | 1.66 | 0.48 |
| Igneous | 60.06 | 0.90 | 15.52 | 3.55 | 4.06 | 0.21 | 3.56 | 5.62 | 3.28 | 2.88 | 0.36 |
| * includes 0.06% BaO and 0.02% SrO. ratios of TiO2/Al2O3: 0.026 (granite); 0.087 (basalt); 0.058 (average igneous rock - basalt:granite 1:1) | |||||||||||
The proximity of the 18th Dynasty dataset to the granite line could suggest that alumina and titania entered the glass system together, perhaps as a consequence of contamination from grinding tools. The proximity of the Ramesside data to the basalt line could suggest that grinding tools of different compositions were used at Dahshur compared to those at Malkata or Amarna. Granite and basalt are both common in Egypt. As hard, igneous rocks have compositions between granite and basalt (i.e. often in a ratio of 65:35, respectively (Mead 1914; Parker 1967)), some variation would be expected between the two lines. However, the absolute levels of Al2O3 appear quite high (~0.5-5%Al2O3) to be explained by contamination from grinding tools alone (i.e. ~30-330g of igneous rock for a 1kg glass ingot), suggesting that another mechanism is responsible for igneous material entering the system.
Similar interactions between TiO2 and Al2O3 are also found with another dataset for Egyptian cobalt-blue glass from Malkata and Amarna measured using LA-ICPMS (Figure 12). Mesopotamian cobalt-blue glass axes recovered from Nippur measured using the same method are also plotted. Both datasets show relatively linear interactions, which again indicates that these two components entered the glass system together. Most of the glass from Nippur falls very close to the granite line while most of the glass samples from Egypt lie mid-way between the basalt and granite lines, i.e. indicative of the average composition of igneous rocks.
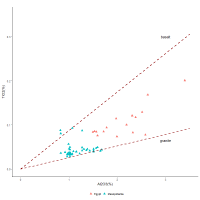
As mentioned above, Egyptian cobalt-blue glass has elevated levels of alumina compared to glasses of other colours, which has been attributed to the alum source. The question that now arises is if titania and alumina entered the glass through contamination during grinding, why would cobalt-blue glass generally have elevated levels of igneous material compared with glass not coloured by cobalt. This will be discussed in Section 4.4. However, regardless of whether grinding or some other mechanism resulted in the elevated levels of alumina and titania in these glass systems, what is apparent is that contamination by the elements found in igneous rocks is sufficient to explain the presence of alumina without recourse to the sophisticated chemistry required to extract cobalt from the cobaltiferous alums. Furthermore, since Mesopotamian cobalt-blue glass is less likely to have been derived from the alum deposits in Egypt, the linearity of the plot supports the premise that the alumina levels for both Egyptian and Mesopotamian glass are related to contamination from igneous rocks. Essentially, there is no need to provenance the cobalt to alum mines in Egypt's Western Oases, which are sedimentary deposits with much higher levels of alumina and lower levels of cobalt than cobalt-blue glass and have not been found to contain titania (Shortland et al. 2006a). Furthermore, without the need to accommodate alumina as part of the suite of elements used to identify the cobalt source, the number of potential sources from where the cobalt derived increases.
Discounting the alum sources of Egypt's Western Desert, in effect, opens up the argument regarding where the cobalt originated. What is clear, however, is that cobalt is associated with Ni, Mn, and Zn, and this suite of elements and the minerals from which they derive is diagnostic of cobalt's source. Moreover, although alumina is probably not part of this mineralisation package, it must still be present in sufficiently high amounts to have entered the system along with these other components, in the same way as the components of gangue enter the slag when metals are extracted from ores.
Internet Archaeology is an open access journal based in the Department of Archaeology, University of York. Except where otherwise noted, content from this work may be used under the terms of the Creative Commons Attribution 3.0 (CC BY) Unported licence, which permits unrestricted use, distribution, and reproduction in any medium, provided that attribution to the author(s), the title of the work, the Internet Archaeology journal and the relevant URL/DOI are given.
Terms and Conditions | Legal Statements | Privacy Policy | Cookies Policy | Citing Internet Archaeology
Internet Archaeology content is preserved for the long term with the Archaeology Data Service. Help sustain and support open access publication by donating to our Open Access Archaeology Fund.